 A collaboration between researchers at UQDI and the Centenary Institute in Sydney has yielded surprising new insight into the way the skin interacts with the immune system. This could have implications on the development of treatments for diseases like inflammatory bowel disease and skin cancer. The findings were published this month in the Proceedings of the National Academy of Sciences.
A collaboration between researchers at UQDI and the Centenary Institute in Sydney has yielded surprising new insight into the way the skin interacts with the immune system. This could have implications on the development of treatments for diseases like inflammatory bowel disease and skin cancer. The findings were published this month in the Proceedings of the National Academy of Sciences.
Normally, when the human body encounters microscopic intruders such as bacteria or viruses, a class of immune cells, called dendritic cells, engulfs the intruder and breaks it down into small parts, called antigens. The dendritic cell then presents those antigens on its surface, and uses them to activate helper T cells, which in turn alert the rest of the immune system to the presence of the intruder. The 'killer' cells in the immune system then seek out and destroy any infected cell that displays the intruder's antigens.
This doesn't seem to be the case when it comes to your skin. Human skin is a microbial ecosystem, where between 500 and 1000 species of harmless bacteria live quite happily. If the immune system were to react to each and every one of these, we would find ourselves in a constant state of inflammation, and our skin destroyed in the process. But why doesn't this happen? Until now, no one really knew.
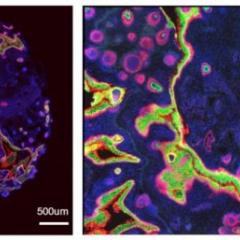
Professor Barbara Fazekas de St Groth at the Centenary Institute suspected it may have something to do with Langerhans cells, the class of dendritic cells found in the skin. She and her team bred a strain of mice whose immune system could only be activated by dendritic cells, then bred another strain whose immune system could only be activated by Langerhans cells. While an immune response was easy to stimulate in the first group of mice, for the Langerhans-only mice, no inflammatory signals triggered an immune response. While the Langerhans cells initially activated T cells, those cells went on to die instead of triggering a wider immune response. In essence, the Langerhans cells were teaching the immune system to tolerate foreign invaders. To find out how, Fazekas de St Groth contacted Professor Ranjeny Thomas and Dr Brendan O’Sullivan at UQDI.
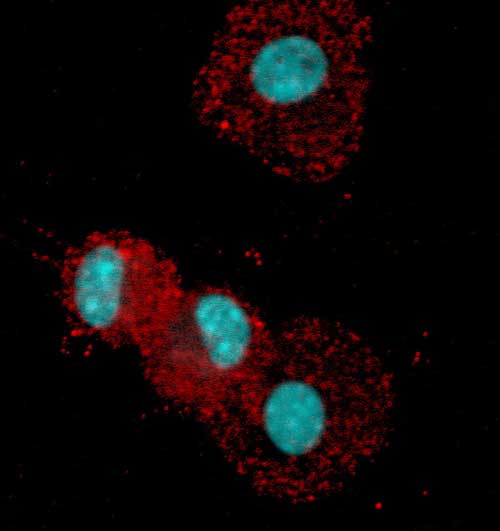
Professor Thomas is well known in the field of immunology for her research into immune tolerance, and her particular interest in a protein called RelB. In dendritic cells, RelB acts as a switch to turn on the genes responsible for activating helper T-cells and telling them to alert other immune cells. Thomas and O’Sullivan discovered that, in order to trigger an immune response, RelB must get to the cell's nucleus, where the genes are stored. Conversely, blocking RelB leads to immune tolerance.
Fazekas de St Groth sent a sample of Langerhans cells to O’Sullivan, who analysed the behavior of RelB in these cells and discovered that, in contrast to dendritic cells, RelB never reaches the nucleus. As a consequence Langerhans cells promote immune tolerance, regardless of the antigen.
Fazekas de St Groth’s discovery represents the first insight into the mechanism that prevents the immune system from attacking the skin. It is also the first evidence of a class of cells completely pre-committed to immune tolerance, and it provides further support for Thomas’ theory that RelB acts as a ‘master regulator’ of dendritic cell function. The implications of these findings are far reaching.
Like the skin, the human gut also contains a microbial ecosystem that our immune system usually tolerates. However, in Inflammatory Bowel Disease (IBD) tolerance is lost and the immune system attacks. This new research will provide fresh insight into the causes of IBD and could aid in the development of new IBD treatments.
This idea is already the basis for Thomas and O’Sullivan’s development of Rheumavax, a vaccine technology aimed at treating Rheumatoid Arthritis (RA). Rheumavax delivers a RelB inhibitor and an antigen associated with RA directly to dendritic cells that can then ‘teach’ the immune system to ignore common triggers of RA. Early stage clinical trials are underway with promising results.
Finally, enabling the immune system to attack the skin might be a good idea. Anti-cancer technologies are emerging that use the immune system to attack cancerous cells. However, the development of such treatments for skin cancer is hampered by the the skin’s innate immune tolerance. Therefore, the outcomes of the UQDI – Centenary Institute collaboration could open the door to treatments that could work together with these immunotherapies in the fight against skin cancer.